Upper limb
Motor impairments are a common consequence after acquired brain injury and a major cause of disability. Specifically, upper limb paresis is among the most significant deficits and represents an important obstacle for independence. Impairment of upper limb motor function is present in more than 80% of stroke survivors, and moderate dexterity after six months is only expected in 30 to 40% of the cases. Recovery of motor function after a brain injury involves neural reorganization of spared areas in both hemispheres to take over functions previously driven by the injured areas. However, reorganization is not driven by mere repetition. It only occurs when experience implies learning. Therefore, it can be deduced that motor rehabilitation should focus on driving plasticity by experiences that mean a challenge for the motor skills of the patients. In addition, motor learning principles, such as intensity, repetition, task-orientation, and feedback have proven to modulate the functional improvement after stroke.
Robot-assisted therapy
Although different meta-analysis have shown that robot-assisted therapy has similar effects to conventional physical therapy when intensity is matched, rehabilitation robotics can increase the intensity of the training.
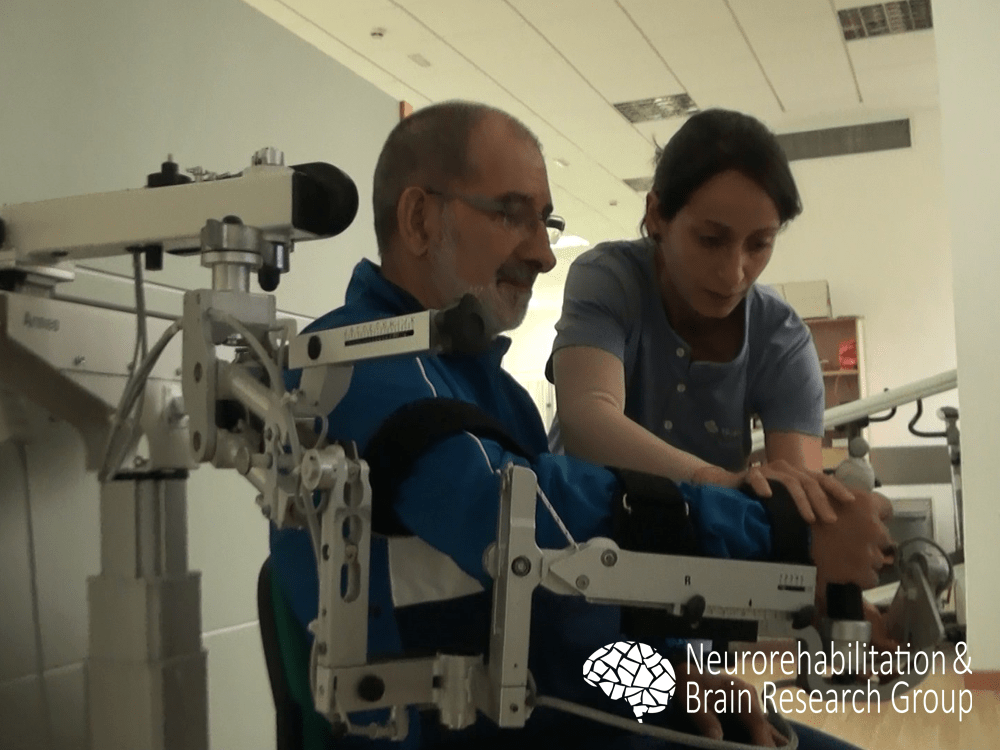
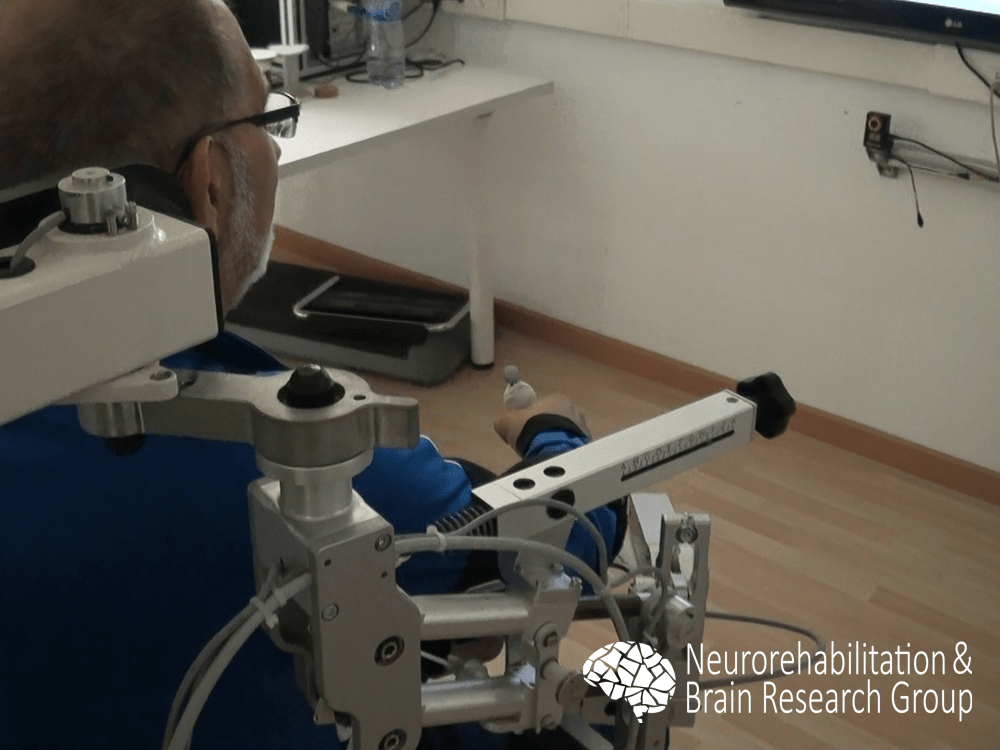
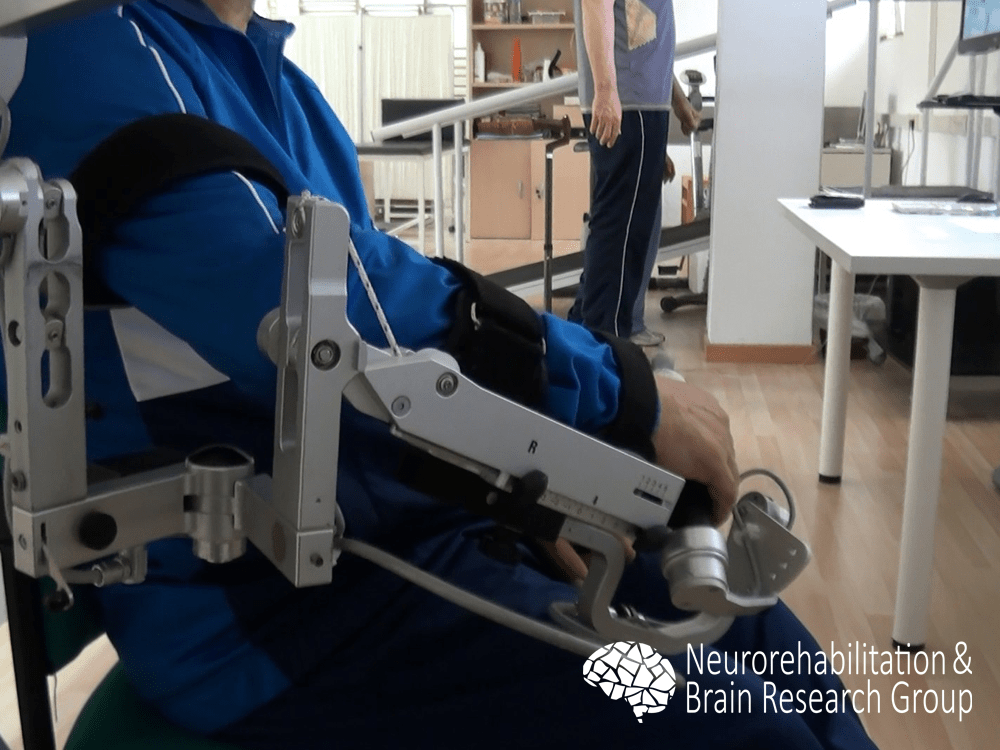
We examined the efficacy of a robot-assisted intervention with the Armeo® Spring (Hocoma, Volketswil, Switzerland) for upper limb rehabilitation in individuals with chronic stroke. Our findings revealed that after an intervention using the robotic system, participants showed significant improvement for all function and activity scales, without significant changes in muscle tone.1 Our results supported previous research in the field, suggesting that robotic-assisted therapy could be combined with physical therapy interventions to enhance the outcomes of upper limb rehabilitation.
Virtual reality-based interventions
Virtual reality is an especially interesting research field since it allows to recreate computer-generated environments and provide customized experiences involving different sensory channels, commonly sight, hearing, and/or touch. An increasing number of studies report promising results of its application to motor rehabilitation after stroke, specifically for upper limb. First, movement kinematics when reaching, grasping, transporting, and releasing objects in a virtual environment are comparable to those in the physical world, thus suggesting that the training of arm movements in virtual reality can be a feasible alternative. Second, virtual reality has been shown effective at improving proximal and global reaching and grasping movements in individuals with stroke in acute and chronic stages. Third, distal fine motor control has also been effectively improved using virtual reality, generally combined with robotic-like devices. Finally, controlled trials suggest that virtual reality may be beneficial to improve upper limb function and performance in activities of daily living, to a greater extent than same dosage of conventional therapy.
Basing on the existing evidence, we have developed a virtual reality-based system that satisfies the motor learning and neural plasticity principles to promote the rehabilitation of task-directed movements of the paretic upper limb. The system fits the motor condition of each subject allowing the training of a wide spectrum of movements, from gross proximal movements to finger dexterity, while being portable and inexpensive, in contrast to robotic systems.
The hardware components of the tabletop system consist of a table, a standard computer, a projector, and a depth sensor, as the Kinect™ (Microsoft, Redmond, WA).2 The projector and the sensor are fixed in an upper plane of the table oriented to its surface. This way, the projector displays the virtual environment on the table and the patients interact within it through movements of their own extremities.
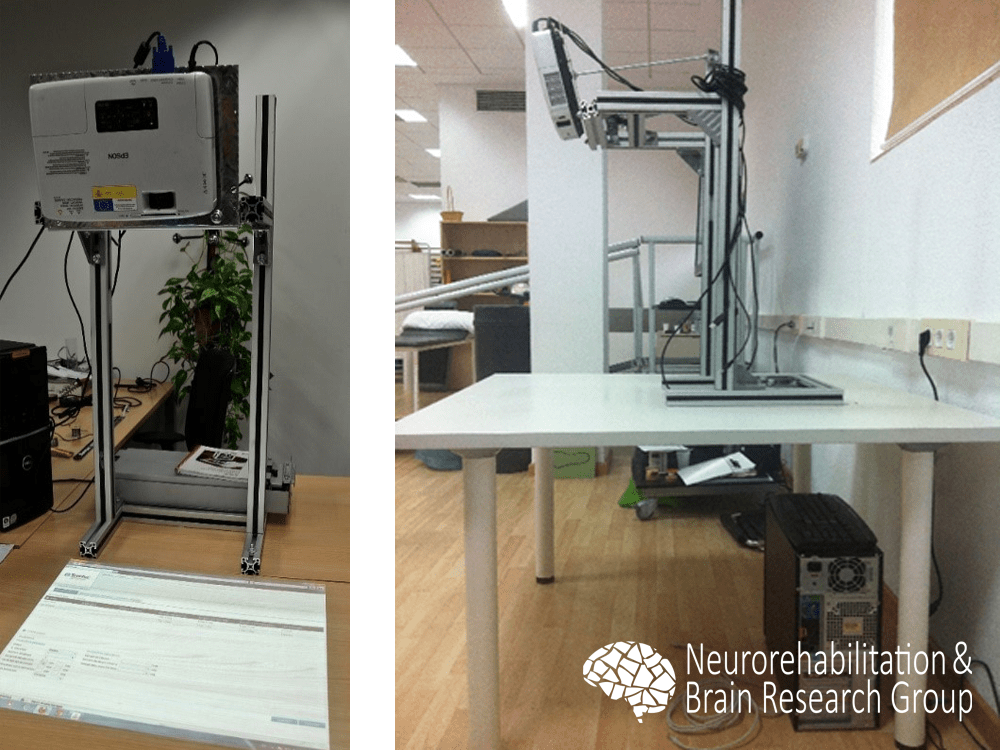
Each exercise requires a specific calibration to define the position in which the implied segments and external objects (if needed) are in contact with the surface. Generally, the segments and objects are estimated using the depth information and their skeletons and their mass centers are defined using the distance matrix of the segments. In those exercises which mostly imply the metacarpophalangeal and interphalangeal joints, a special effort is done to track the fingers. In this case, the hand is segmented likewise and then the convex hull is applied to estimate the fingers.
With the described tracking capabilities we developed a set of exercises that cover movements that were likely to belong to the motor repertory of the patients previously to the injury, aiming to maximize the correlation of the virtual tasks with the real ones. For instance, the objectives of some exercises are to dial a telephone number, to cook, to knock a door, etc.
To examine the clinical efficacy of the system we enrolled individuals with chronic stroke in a reversal (A-B-A) study.3,4 Phase A involved conventional physical therapy, and phase B involved an experimental intervention with the virtual reality-based system. Although no significant differences were detected in either the body structures or functions, participants showed a significant improvement in the body activities after the whole intervention, significantly promoted by the virtual reality-based intervention (Phase B). However, this improvement was detected neither after the previous nor the following physical therapy intervention.
Our results support the clinical effectiveness of virtual reality-based interventions that satisfy the motor learning principles for upper limb rehabilitation in chronic stroke survivors. This characteristic, together with the low cost of the system, its portability, and its acceptance could promote the integration of these systems in the clinical practice as an alternative to more expensive systems, such as robotic instruments.
Mirror therapy
Robotic-assisted and virtual reality-based interventions require residual motor function to be included in rehabilitation protocols. Then, what therapeutic options have those individuals with severe paresis, which impedes active training of the arm and hand? Traditionally, rehabilitation strategies in these subjects have almost exclusively aimed to compensate for the deficit by training the opposite limb in daily tasks. As synaptic connectivity is highly use dependent, this absence of stimulation on the chronic paretic arm results in reduced sensorimotor representation in the available neural circuits over time and consequently diminishes the possibilities for sensorimotor clinical progress. Accordingly, a reduction in sensorimotor abilities might partly be an effect of non-use of the affected limb. Mirror therapy has been posed to address these issues because it does not require active movements of the affected arm.
Mirror therapy uses a mirror to occlude the affected arm and reflect the movements of the non-affected arm, thus tricking the brain to feel that the movement is performed with the affected arm.
Preliminary results with phantom limbs syndrome and pain management have motivated the application of mirror therapy to sensorimotor rehabilitation after stroke. Previous research has shown that this therapy can improve the motor function of chronic individuals with stroke with mild to moderate impairment. Moreover, interventions involving stroke survivors with severe upper limb paresis have been shown to provide limited motor improvement in the acute or sub-acute phase. However, no previous research has described the effects of mirror therapy in chronic individuals with stroke with severely impaired upper limb function. To examine this, we enrolled chronic subjects post-stroke with severely impaired upper limb function, who were randomly involved in a passive mobilization program or mirror therapy.5
After the clinical trial, an improvement in motor function was observed in both groups on time and ability, while no differences were detected in kinesthesis or stereognosis. However, individuals who underwent the mirror therapy showed a significant improvement in tactile sensation that was mainly observed as an increased sensitivity to light touches. Consequently, in comparison with passive mobilization, mirror therapy in chronic stroke survivors with severely impaired upper-limb function may provide a limited but positive effect on light touch sensitivity while providing similar motor improvement.
References
- Colomer C., Baldoví A., Torromé S., Navarro M.D., Moliner B., Ferri J., Noé E. Efficacy of Armeo® Spring during the chronic phase of stroke. Study in mild to moderate cases of hemiparesis. Neurología. 2013. 28(5):261-267.
- Llorens R., Marín C., Ortega M., Alcañiz M., Colomer C., Navarro M.D., Noé E. Upper limb tracking using depth information for rehabilitative tangible tabletop systems. 9th Intl Conf. Disability, Virtual Reality & Associated Technologies, 2012. 463-466.
- Llorens R., Colomer C., Noé E., Ortega M., Alcañiz M. Functional improvement of hemiparetic upper limb after a virtual reality-based intervention with a tabletop system and tangible objects. 10th Intl Conf. on Disability, Virtual Reality and Assoc. Technologies, 2014, pp. 99-106.
- Colomer C., Llorens R., Noé E., Alcañiz M. Effect of a mixed reality-based intervention on arm, hand, and finger function on chronic stroke. Journal of NeuroEngineering andRehabilitation, 2016, 13:45.
- Colomer C., Noé E., Llorens R. Mirror therapy in chronic stroke survivors with severely impaired upper limb function: A randomized controlled trial. European Journal of Physical and Rehabilitation Medicine, 2016. Jun; 52(3):271-8.